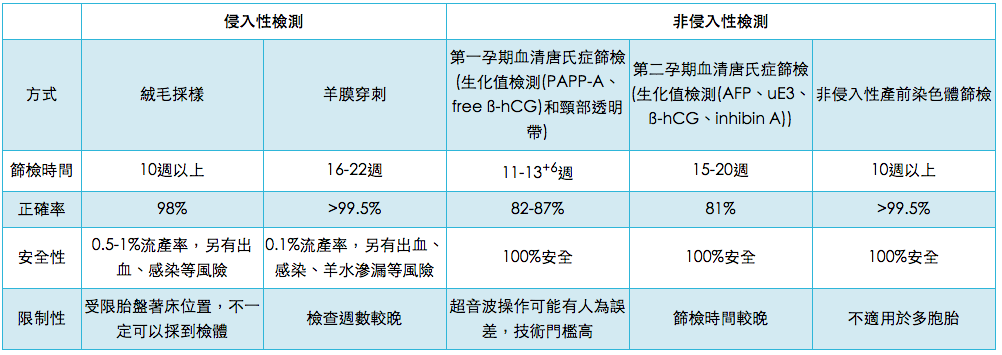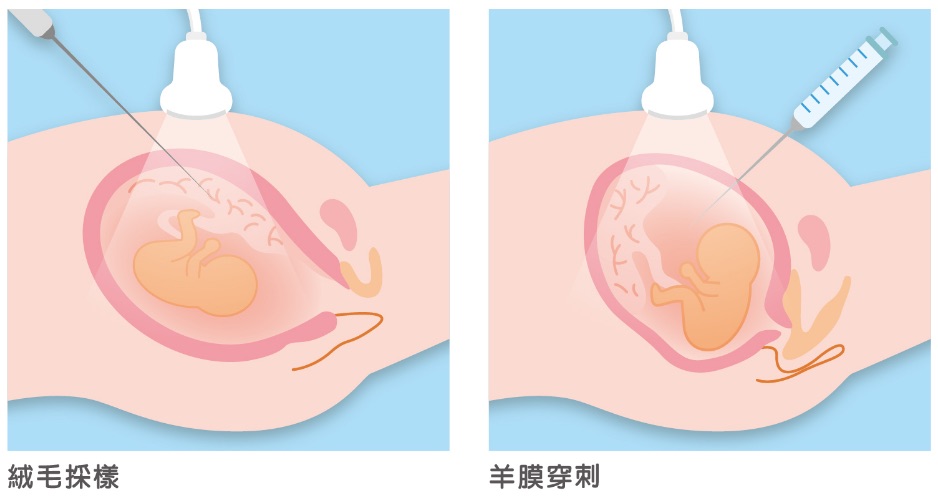
Chorionic Villus Sampling (CVS) has been used as an alternative early-stage diagnostic option since 1983. It is recommended that Chorionic Villus Sampling (CVS) be performed during the 10th week of pregnancy to avoid increasing the risk of body malformations. Unfortunately, because the sample is collected from placental tissue, this procedure has a possibility of yielding false results. Chorionic Villus Sampling (CVS) and amniocentesis test for similar medical conditions; however, Chorionic Villus Sampling (CVS) is preferred for hereditary diseases which can only be detected by testing a greater amount of DNA.
Research conducted in 1997 discovered Y-chromosome fragments in the blood of a woman who was carrying a male child. This finding revealed that it was possible to retrieve fetal chromosomes from the mother's blood and test them for Down syndrome. In 2005, such techniques were made commercially available.Today, they are not only simple and easy, but also boast a 99% accuracy level.
Thanks to improvements in techniques, the risk of Chorionic Villus Sampling (CVS) or amniocentesis causing complications has greatly decreased, and risk is particularly low when an experienced physician performs the procedure. Currently, the miscarriage rate associated with Chorionic Villus Sampling (CVS) is around 1%. However, in some cases, sampling difficulties (e.g. due to misplaced placenta) or insufficient sample size can affect results.
For amniocentesis, the miscarriage rate is around 0.5-1.4%; the risk of hypertonic uterine dysfunction, colporrhagia or leakage of amniotic fluid is around 2-3%; and the risk of more serious complications is around 0.2-0.5%. In addition, approximately 1% of pregnant women suffer from early stage water breaks within 48 hours of amniocentesis. Other research has found that amniotic fluid fails to be collected in around 1% of cases, and approximately 2% of women must undergo a second procedure as a result.
Before traditional karyotyping is performed, both Chorionic Villus Sampling (CVS) and amniocentesis require that the sample be cultivated in the laboratory for a certain period of time. The entire process takes around 2-3 weeks.